The removal of dissolved CO2 from natural gas is essential for the safe and reliable operation of liquefied natural gas (LNG) systems. The purification of natural gas (NG) from CO2 down to a concentration of 50 ppm by multi-stag distillation is theoretically investigated. A three-column distillation system is proposed that can purify NG to lower than 50 ppm concentration of CO2, while avoiding CO2 freezout. The columns include a 30-stage demethanizer, in which high purity methane is obtained in the distillate by separating the impurities from natural gas including CO2; a 50-stage extractive column where the azeotrope between CO2 and ethane is broken; and a 50-stage solvent recovery column that recovers a mixture of heavy hydrocarbons suitable for recycling as a solvent back into the extractive column. The proposed system can operate in a closed loop arrangement where the bottoms stream that leaves the recovery column can be recycled and injected.
There are two major technical challenges in the distillation-based removal of CO2 from natural gas. The first problem is that the CO2 freezes out in the demethanizer distillation column. CO2 exists primarily as vapor-solid phase at typical demethanizer conditions. Significant research has been done on the CO2-CH4 phase equilibrium. A Ryan-Holmes approach is adopted in this work where a multicomponent feed containing heavier hydrocarbons is used in the demethanizer column.
The second major problem associated with distillation-based removal of CO2 from NG is that CO2 and ethane, the second largest constituents of NG after methane, form an azeotrope in the bottom streams of distillation system. This azeotrope must be broken. The ease of separation by distillation is closely related to relative volatility, which is a measure of the effective vapor pressure ratio of the key components that need to be separated. The number of stages and therefore the capital cost can be very high if the relative volatility is close to unity, as is the case for an azeotrope. Keeping into account the capital investment and operating costs, relative volatility values greatly different from unity are desired. When the mixture consists of species where relative volatilities are at or near unity separation cannot occur by fractional distillation. Extractive distillation and azeotropic distillation methods are then used where a component called solvent or entrainer is used to alter the relative volatilities of the key components.
The objective of this investigation is to examine the feasibility of a multi-stage distillation based CO2 removal system for LNG, where freeezeout and azeotropes are avoided, and to develop and theoretically demonstrate a multi-tower pseudo-closed loop distillation system with solvent recovery which can be recycled back into the system. This investigation is novel, as a complete and functional cryogenic distillation-based CO2 purification system has not been published in the open literature.
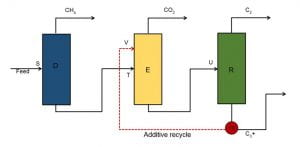
Figure 1 displays the schematic of a three column system that meets the aforementioned objectives of this investigation. The first column denoted by D represents the demethanizer column, which essentially removes all the methane from the mixture. The feed (natural gas) comprises of a multicomponent mixture of hydrocarbons like C3, iC4, nC4, iC5 and nC5. The feed natural gas is assumed to be 85% methane in this study and the remainder is assumed to be a mixture of heavier hydrocarbons and carbon dioxide occurring naturally in most reserves. Freezeout analysis is done at every stage to ensure smooth operation and thus 28 pseudo streams in between the top and bottom “real” streams are employed. The second column denoted by E in Figure 1 is the Extractive column. The recovery column is denoted by R and has a total of 50 stages.
